Abstract
Background: Polatuzumab vedotin is an antibody-drug conjugate targeting CD79b on malignant B-cells. Polatuzumab vedotin in combination with bendamustine and rituximab (pola-BR) improved complete response (CR) rate and overall survival (OS) compared with BR alone in patients (pts) with relapsed/refractory diffuse large B-cell lymphoma (R/R DLBCL; Sehn et al. J Clin Oncol 2020). Several treatment options for pts with R/R DLBCL are recommended by the National Comprehensive Cancer Network (NCCN) guidelines (2021), including platinum-based chemotherapies such as oxaliplatin in combination with rituximab and gemcitabine (R-GemOx). Adding polatuzumab vedotin to R-GemOx (pola-R-GemOx) may improve outcomes for pts where an unmet need still exists. However, the safety of polatuzumab vedotin in combination with platinum-based therapies must be considered, as both are associated with neuropathy. The ongoing POLARGO study is assessing the safety and efficacy of pola-R-GemOx compared with R-GemOx alone in pts with R/R DLBCL who are ineligible for stem cell transplant (SCT).
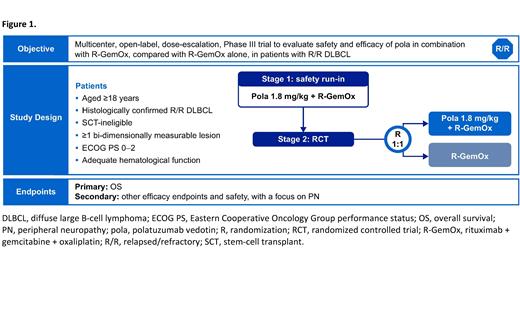
Methods: POLARGO (NCT04182204; MO40598) is a multicenter, open-label, Phase III study, comprising a safety run-in stage (pola-R-GemOx; n=10) and a randomized controlled trial (RCT) stage (pola-R-GemOx vs R-GemOx; n=206 pts; Figure 1). In the RCT stage, pts will be recruited from 80─90 sites globally.
Pts must be aged ≥18 years, have received at least one line of prior systemic therapy, have histologically confirmed R/R DLBCL, and for the RCT stage, have confirmed availability of archival or freshly collected tumor tissue. Relapse is defined as a recurrence of disease following a response lasting six months or more after completion of the last line of therapy; refractory is disease that progressed during prior therapy or progressed within six months after completion of the last line of therapy. Patients are also required to have an Eastern Cooperative Oncology Group performance status (ECOG PS) of 0-2, and adequate hematologic function. Exclusion criteria include prior allogeneic SCT, planned autologous/allogeneic SCT, and baseline peripheral neuropathy greater than Grade 1 (as assessed by National Cancer Institute Common Terminology Criteria for Adverse Events, Version 5.0 [NCI CTCAE v5.0]).
The primary endpoint of the safety run-in stage is the safety and tolerability of polatuzumab vedotin (1.8 mg/kg) + R-GemOx (R, 375 mg/m 2; Gem, 1000 mg/m 2; Ox, 100 mg/m 2) administered in 21-day cycles. Safety will be assessed by recording the incidence, nature, and severity of AEs (NCI CTCAE v5.0), with a focus on peripheral neuropathy. Dose interruptions, reductions, and intensity will be used to determine tolerability. Administration of granulocyte-colony stimulating factor, as a primary prophylaxis, is required in each cycle of therapy.
In the RCT stage, pts will be stratified by their number of previous lines of systemic therapy for DLBCL, outcome of last systemic therapy (relapsed vs refractory), and age (≤70 years vs >70 years). Pts will be randomized (1:1) to receive up to eight 21-day cycles of either pola-R-GemOx or R-GemOx alone.
The primary efficacy endpoint of the RCT stage is OS, defined as the time from randomization to death from any cause. Key secondary endpoints include CR and objective response rate assessed by an independent review committee, and progression-free survival, duration of objective response, and event-free survival assessed by investigator, according to Lugano 2014 response criteria.
Positron emission tomography-computed tomography (PET-CT) and CT scans will be obtained at screening, during, and after the treatment period. The impact of treatment on health-related quality of life will also be assessed. Patient follow-up will continue for up to 2 years.
As of July 07, 2021, the safety run-in stage has completed enrollment with a total of 13 pts, and the RCT stage is planned to open in November 2021.
Matasar: GlaxoSmithKline: Honoraria, Research Funding; Merck Sharp & Dohme: Current holder of individual stocks in a privately-held company; Genentech, Inc.: Consultancy, Honoraria, Research Funding; Merck: Consultancy; Teva: Consultancy; Janssen: Honoraria, Research Funding; Juno Therapeutics: Consultancy; Pharmacyclics: Honoraria, Research Funding; Daiichi Sankyo: Consultancy; ImmunoVaccine Technologies: Consultancy, Honoraria, Research Funding; Rocket Medical: Consultancy, Research Funding; IGM Biosciences: Research Funding; Takeda: Consultancy, Honoraria; TG Therapeutics: Consultancy, Honoraria; Memorial Sloan Kettering Cancer Center: Current Employment; Seattle Genetics: Consultancy, Honoraria, Research Funding; F. Hoffmann-La Roche Ltd: Consultancy, Honoraria, Research Funding; Bayer: Consultancy, Honoraria, Research Funding. Haioun: Gilead: Honoraria, Research Funding; Celgene: Honoraria, Research Funding; Amgen: Honoraria, Research Funding; Janssen: Honoraria, Research Funding; Novartis: Honoraria, Research Funding; F. Hoffmann-La Roche Ltd: Honoraria, Research Funding; Servier: Honoraria, Research Funding; Takeda: Honoraria, Research Funding; Miltenyi: Honoraria, Research Funding. Sancho: Takeda: Honoraria; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bristol-Myers-Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees; Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; F. Hoffmann-La Roche Ltd: Honoraria, Membership on an entity's Board of Directors or advisory committees; Incyte: Membership on an entity's Board of Directors or advisory committees. Viardot: Amgen: Membership on an entity's Board of Directors or advisory committees; University Hospital of Ulm: Current Employment; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bristol-Myers Squibb: Honoraria, Membership on an entity's Board of Directors or advisory committees; Kite/Gilead: Honoraria, Membership on an entity's Board of Directors or advisory committees; F. Hoffmann-La Roche Ltd: Honoraria, Membership on an entity's Board of Directors or advisory committees. Hirata: Genentech, Inc.: Current Employment; F. Hoffmann-La Roche Ltd: Current holder of stock options in a privately-held company. Perretti: F. Hoffmann-La Roche Ltd: Current Employment. Musick: F. Hoffmann-La Roche Ltd/Genentech, Inc.: Current Employment, Current holder of stock options in a privately-held company. McMillan: Pfizer: Research Funding; F. Hoffmann-La Roche Ltd: Consultancy, Honoraria, Speakers Bureau; Amgen: Membership on an entity's Board of Directors or advisory committees; Abvie: Membership on an entity's Board of Directors or advisory committees.
Polatuzumab vedotin is an antibody-drug conjugate targeting CD79b on malignant B-cells. Polatuzumab vedotin in combination with bendamustine and rituximab (pola-BR) improved complete response (CR) rate and overall survival (OS) compared with BR alone in patients (pts) with relapsed/refractory diffuse large B-cell lymphoma. Pola-BR is approved for the treatment of adult patients with relapsed or refractory diffuse large B-cell lymphoma, not otherwise specified, after at least two prior therapies.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal